The Concept and Study On the Properties of Mixture
This post was last updated on April 6th, 2024
We come across a variety of things labelled as “pure” in our daily lives. Milk, butter, ghee, and other products’ packaging would have had the message. For us, what does “pure” mean? We may use the phrase “pure” to something that is not tainted or contaminated by other substances, yet this is not the case in scientific terms. Pure substances are defined as those that contain just one ingredient.
As a result, even though the milk in the carton is labelled as “pure,” it is, in fact, not. It is not recognised to be a pure substance by science but rather a combination.
Let’s take a closer look at the many forms of mixes.
Contents
ToggleWhat Is a Mixture?
The term “mixture” refers to a material that combines two or more distinct kinds. It is possible to separate them by using physical measures. Examples include a saltwater solution, a sugarwater solution, different gases, and the atmosphere itself. There are no chemical changes that link the components of any combination, and a consequence of this is that each component retains its features.
To make matters worse, unlike in a compound, the constituents of a mixture do not interact with chemical mixtures to create new material. As a result, they just Mixture and retain their distinct features. The lemonade seen above is a mixture since the ingredients aren’t all in the same proportions.
Lemonade may be prepared with more or less lemon juice or with more or less sugar and still be referred to as lemonade despite the differences.
Mixtures have several key characteristics, such as:
- Components or substances in a mixture maintain their original physical qualities when they are combined.
- We may utilise several techniques to separate the mixture into its components physically.
- A mixture’s components may or may not be in a set ratio and may or may not be in the same amount.
The following are some examples of common mixtures:
- Water and sugar
- Sodium chloride with water
- Atmosphere ( mixture of gases),
- Sugar and salt, in that order.
- Water and sand
- Oil and water, and so forth.
- Mixtures of many kinds are listed here.
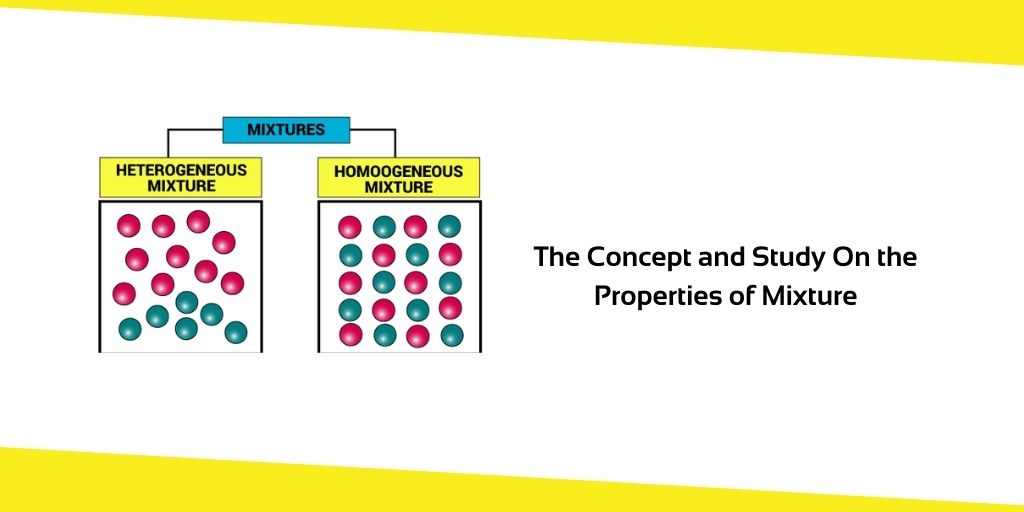
There are two primary sorts of mixtures. These are some of the things.
- Homogeneous Mixtures
- Heterogeneous Mixtures
In both homogeneous and heterogeneous mixtures, the distribution of the components.
Homogeneous Mixtures:
In Latin, “homo” refers to sanity. Homogeneous mixes are those in which the constituents are evenly dispersed throughout. When it comes to salt and water, for example, the flavour of the water is the same no matter where you drink from it. Because salt is evenly dispersed throughout the mixture, this demonstrates that it is.
Examples include salt and water, sugar and water, alcohol and water, and so on.
- The properties of homogeneous mixtures.
- Components are evenly dispersed throughout the mixture.
- Components cannot be separated by centrifugal force.
When a light beam is an incident on a homogeneous mixture, it does not display the Tyndall effect, which is the scattering of light by the particles in the medium. It is because of the dispersion of the light beam that the path of light is apparent. The particle size is less than a nanometer.
All of the mixes are completely homogenous.
Heterogeneous Mixtures:
The term “hetero” denotes “different.” There are heterogeneous mixes when the components are dispersed in a non-uniform fashion across the mixture. Sand and water, for example, is an example of a heterogeneous combination since sand is not evenly distributed in the water. For example, sand, sugar, salt or ice in the water.
Heterogeneous Mixtures: Their Properties
- The components of a heterogeneous mixture are not distributed evenly across it.
- Just by looking at the combination, you can tell the components apart.
- Its particle size varies from 1 nm to 1 m in diameter.
- They may show the Tyndall effect.
- Based on particle size, the solutions may be further subdivided into three categories.
Listed below are the details:
These are homogenous mixes, which is what they are. Solutions have a particle size of less than one nanometer. Centrifugation and decantation are unable to separate the solutions into their constituent parts. Air, sugar and water, salt and water, and so on are examples of this.
From 1 nanometer to 1 micrometre in diameter, colloids are a kind of aggregation. A microscope is the only way to view the distinct components, which can only be seen with the naked eye. An illustration of this would be fog or smoke.
More than 1 micrometre in particle size is required for suspensions.
Brownian Motion describes the zig-zag motion of suspension components. Suspensions’ stabilising agents keep their constituent parts separate and safe from one other. When stabilising chemicals aren’t present, the Suspension breaks down into its constituent parts on its own. An example is milk, cream, butter, and so on.
Solved Examples:
Q 1. What exactly do you mean when you say “homogeneous mixtures”?
Answer:
All of the components of a homogeneous mixture are evenly dispersed throughout the mixture. Sugar and water, salt and water, and so on are examples.
Q 2. Heterogeneous mixes are a kind of mixture.
Answer:
All of the parts aren’t uniformly distributed, and the mixture isn’t homogeneous. Certain places in the mixture may have a high or low concentration of a certain ingredient, and examples include sand and water, salt and sand, and so forth.
Q 3. Explain the Tyndall effect in question three.
Answer:
The dispersion of light occurs when a beam of light is directed towards a medium’s particles. The path of light is seen as a consequence of dispersion.
Q 4. Solution, colloids, and suspensions all have different particle sizes.
Answer:
Solutions, colloids, and suspensions all have the same particle size:
- 1 nm
- Colloids: 1 nm to 1 m
- >1 m Suspensions are required.
Q 5. What are Brownian particles?
Answer:
Brownian Particles suspended in a suspension move in a random fashion. In the medium, particles travel in a random zig-zag pattern.
Q 6. What exactly is a pure material, and how can we define it?
Answer:
Particles of a single element or combination make up a pure material. As an illustration: a variety of salts and sugars.
Conclusion
This article will provide students with a better understanding of the world of chemistry, especially understanding mixtures. Thoroughly go through this article to grasp the knowledge of mixture and its various components.
Recommended For You
UPSC CDS 2021 – Is it possible to clear UPSC CDS in first attempt?
Most Inside
Most Inside offers high-quality recommendations and valuable updates to enhance all aspects of your life, providing premium guidance and enriching experiences.




